Reckitt Benckiser, the makers of Nurofen Plus, a pain medicine found in the U.K. have said the boxes of Nurofen Plus tablets containing AstraZeneca's antipsychotic Seroquel XL is "suspected sabotage", according to the company press release. Pfizer's anti eptileptic medicine Neurontin was also found in at least one of the packages in Northern Ireland according to the press release from the MHRA.
Reckitt Benckiser Press Release
Nurofen Plus product recall
The following notice has been issued by the manufacturer:
Nurofen Plus Ibuprofen, Codeine tablets. Pack sizes of 12’s, 16’s, 24’s, 32’s
Nurofen Plus is being recalled. Consumers are being asked to return packs of Nurofen Plus to their nearest pharmacy following 5 cases of other manufacturers medicine being found in boxes of Nurofen Plus. The safety of our consumers is paramount. Even though there has been no serious health consequences to any consumer, we will not take any risk regarding the quality or safety of our products. No other products from the Nurofen range are affected.
Sabotage is suspected and we are working with the police on a formal investigation to find the person or persons responsible. Distribution of Nurofen Plus has been halted at this time.
This decision has been taken in full consultation with the Medicines & Healthcare products Agency (MHRA) as a precautionary measure. Consumers are advised to return any packs of Nurofen Plus to any pharmacy where a refund will be provided. Pharmacists are advised to return stock to their wholesaler from where it will be collected.
We would like to apologise to our consumers & customers for any inconvenience caused, and thank them in advance for their cooperation. If more information is required, please call us on 0500 455 456."
AstraZeneca U.K. company press release:
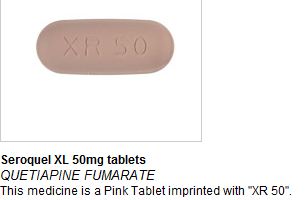
On Thursday 25th August MHRA issued a drug alert to all pharmacists and healthcare trusts after reports of Seroquel XL 50mg tablets (quetiapine prolonged release), an atypical antipsychotic medication, being found in a small number of boxes of Nurofen Plus. Following additional reports of Seroquel XL and another (non-AstraZeneca) product being found in further batches of Nurofen Plus, a recall of all Nurofen Plus tablets has now been issued by the manufacturer.
It should be made clear that Seroquel XL is not affected by this recall and patients should continue to take their Seroquel XL as prescribed. As with all prescription medicines, patients who have any concerns about Seroquel XL should contact their doctor or pharmacist.
In affected Nurofen Plus packs, it appears that the standard blister strips of Nurofen Plus tablets have been removed and replaced with foil sealed blister strips of Seroquel XL 50mg tablets. The strips are clearly labelled as Seroquel XL.
Seroquel XL tablets and Nurofen are made by two different companies (AstraZeneca & Reckitt Benckiser) and are not manufactured or packaged in the same factory. Manufacturing errors by Reckitt Benckiser and AstraZeneca are not considered to be part of the cause at this stage. There is also no indication to date that any countries are affected beyond the UK.
Patient safety is the primary concern of AstraZeneca and the company is taking this issue very seriously. AstraZeneca is supporting the MHRA and Reckitt Benckiser in the investigation of the cause.
For information about the recall or drug alert, or the investigation into the cause of this issue, please contact the MHRA press office.
For medical information enquiries related to Seroquel XL please contact AstraZeneca Ltd medical information department on 0800 783 0033.
For medical information enquiries related to Nurofen Plus Tablets please contact Reckitt Benckiser (UK) Ltd medical information department on 0500 455 456.
For medical information enquiries related to Neurontin 100mg capsules, which have also now been found in one batch of Nurofen Plus, please contact Pfizer medical information department on 01304 616161."
MHRA press release: (update below)
We are advising people to return all packets of Nurofen Plus pain relief tablets to any pharmacy following a recall of this medicine by the licence holder Reckitt Benckiser (UK) as a precautionary measure. The medicine was recalled after reports were received that the anti-psychotic drug Seroquel XL 50mg had been found in four packets in South London. A further packet containing Neurontin 100mg, a medicine used to treat epilepsy, was found in Northern Ireland.
If you have purchased a packet please check the contents carefully and exercise caution.
If you think you may have taken a Seroquel or Neurontin tablet and have any concerns please speak to your healthcare professional.
Is my packet affected?
Seroquel tablets are large and capsule shaped and can be identified by their gold and black packaging.
Nurofen Plus tablets are smaller and can be identified by their silver and black packaging.
Neurontin tablets are white capsules, printed with name of the drug and containing a white to off-white powder."
News article
Nurofen Plus Recalled; Sabotage Suspected-PharmTech:
"The UK's Medicines and Healthcare products Regulatory Agency (MHRA) issued several press releases Aug. 25–26, 2011, regarding the discovery of rogue medicines in packs of Reckitt Benckiser’s Nurofen Plus.
The most recent of the press releases announces that Reckitt Benckiser (UK) Ltd is to recall all remaining unexpired stock of Nurofen Plus tablets of any pack size as a precautionary measure. The discovery of AstraZeneca's anti-psychotic drug Seroquel XL in Nurofen Plus packs on Aug. 25, 2011 was followed by a pack containing Pfizer's Neurontin 100mg, used to treat epilepsy, being found in Northern Ireland on Aug. 26, 2011.
The MHRA advised the public in a previous press release to return any packs of Nurofen Plus pain relief tablets to any pharmacy. The release also indicated the differences between the blister packs and medicines contained within. Seroquel tablets are large and capsule shaped in gold and black packaging, Nurofen Plus blister packs are silver and black, and Neurontin tablets are white capsules printed with name of the drug. Ian Holloway from the MHRA's Defective Medicines Report Centre (DMRC) said, "People should check to see if they have any packets of Nurofen Plus. If you do, return them to your nearest pharmacy."
Reckitt Benckiser issued the recall on the Nurofen website and stated, "Sabotage is suspected and we are working with the police on a formal investigation to find the person or persons responsible. Distribution of Nurofen Plus has been halted at this time."
NUROFEN PLUS NOW IN FULL RECALL 8-31-11
Drug alert: Nurofen Plus tablets update
Tue, 30/08/2011 - 11:32
By News team
All unexpired stock of Nurofen Plus tablets are now being recalled following further reports of Seroquel XL and Neurontin blister strips being found in cartons of Nurofen Plus.
The Medicines and Healthcare products Regulatory Agency issued a class 1 (patient level) drug alert on Friday evening (26 August 2011) following a recall by Reckitt Benckiser. The company has received further reports of two additional batches of Nurofen Plus tablets affected — one of the batches contained Seroquel XL tablets 50mg and one contained the Pfizer product Neurontin capsules 100mg.
Previous advice by the MHRA has been updated and Reckitt Benckiser is now recalling all remaining unexpired stock of Nurofen Plus tablets in any pack size as a precautionary measure. The MHRA is advising the public to return packs to pharmacies.
Enquiries related to Seroquel XL from AstraZeneca medical information on 0800 783 0033.
Enquiries related to Neurontin from Pfizer medical information on 01304 616161.
Enquiries related to Nurofen Plus from Reckitt Benckiser medical information department on 0500 455 456.
*This is only for the U.K.
No comments:
Post a Comment
Please be civil, agree to disagree, and express your opinion. Comments will be moderated for content to avoid inflammatory language and spam