Reckitt Benckiser, the makers of Nurofen Plus, a pain medicine found in the U.K. have said the boxes of Nurofen Plus tablets containing AstraZeneca's antipsychotic Seroquel XL is "suspected sabotage", according to the company press release. Pfizer's anti eptileptic medicine Neurontin was also found in at least one of the packages in Northern Ireland according to the press release from the MHRA.
Reckitt Benckiser Press Release
Nurofen Plus product recall
The following notice has been issued by the manufacturer:
Nurofen Plus Ibuprofen, Codeine tablets. Pack sizes of 12’s, 16’s, 24’s, 32’s
Nurofen Plus is being recalled. Consumers are being asked to return packs of Nurofen Plus to their nearest pharmacy following 5 cases of other manufacturers medicine being found in boxes of Nurofen Plus. The safety of our consumers is paramount. Even though there has been no serious health consequences to any consumer, we will not take any risk regarding the quality or safety of our products. No other products from the Nurofen range are affected.
Sabotage is suspected and we are working with the police on a formal investigation to find the person or persons responsible. Distribution of Nurofen Plus has been halted at this time.
This decision has been taken in full consultation with the Medicines & Healthcare products Agency (MHRA) as a precautionary measure. Consumers are advised to return any packs of Nurofen Plus to any pharmacy where a refund will be provided. Pharmacists are advised to return stock to their wholesaler from where it will be collected.
We would like to apologise to our consumers & customers for any inconvenience caused, and thank them in advance for their cooperation. If more information is required, please call us on 0500 455 456."
AstraZeneca U.K. company press release:
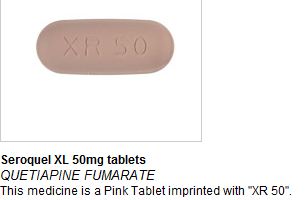
On Thursday 25th August MHRA issued a drug alert to all pharmacists and healthcare trusts after reports of Seroquel XL 50mg tablets (quetiapine prolonged release), an atypical antipsychotic medication, being found in a small number of boxes of Nurofen Plus. Following additional reports of Seroquel XL and another (non-AstraZeneca) product being found in further batches of Nurofen Plus, a recall of all Nurofen Plus tablets has now been issued by the manufacturer.
It should be made clear that Seroquel XL is not affected by this recall and patients should continue to take their Seroquel XL as prescribed. As with all prescription medicines, patients who have any concerns about Seroquel XL should contact their doctor or pharmacist.
In affected Nurofen Plus packs, it appears that the standard blister strips of Nurofen Plus tablets have been removed and replaced with foil sealed blister strips of Seroquel XL 50mg tablets. The strips are clearly labelled as Seroquel XL.
Seroquel XL tablets and Nurofen are made by two different companies (AstraZeneca & Reckitt Benckiser) and are not manufactured or packaged in the same factory. Manufacturing errors by Reckitt Benckiser and AstraZeneca are not considered to be part of the cause at this stage. There is also no indication to date that any countries are affected beyond the UK.
Patient safety is the primary concern of AstraZeneca and the company is taking this issue very seriously. AstraZeneca is supporting the MHRA and Reckitt Benckiser in the investigation of the cause.
For information about the recall or drug alert, or the investigation into the cause of this issue, please contact the MHRA press office.
For medical information enquiries related to Seroquel XL please contact AstraZeneca Ltd medical information department on 0800 783 0033.
For medical information enquiries related to Nurofen Plus Tablets please contact Reckitt Benckiser (UK) Ltd medical information department on 0500 455 456.
For medical information enquiries related to Neurontin 100mg capsules, which have also now been found in one batch of Nurofen Plus, please contact Pfizer medical information department on 01304 616161."
MHRA press release: (update below)
We are advising people to return all packets of Nurofen Plus pain relief tablets to any pharmacy following a recall of this medicine by the licence holder Reckitt Benckiser (UK) as a precautionary measure. The medicine was recalled after reports were received that the anti-psychotic drug Seroquel XL 50mg had been found in four packets in South London. A further packet containing Neurontin 100mg, a medicine used to treat epilepsy, was found in Northern Ireland.
If you have purchased a packet please check the contents carefully and exercise caution.
If you think you may have taken a Seroquel or Neurontin tablet and have any concerns please speak to your healthcare professional.
Is my packet affected?
Seroquel tablets are large and capsule shaped and can be identified by their gold and black packaging.
Nurofen Plus tablets are smaller and can be identified by their silver and black packaging.
Neurontin tablets are white capsules, printed with name of the drug and containing a white to off-white powder."
News article
Nurofen Plus Recalled; Sabotage Suspected-PharmTech:
"The UK's Medicines and Healthcare products Regulatory Agency (MHRA) issued several press releases Aug. 25–26, 2011, regarding the discovery of rogue medicines in packs of Reckitt Benckiser’s Nurofen Plus.
The most recent of the press releases announces that Reckitt Benckiser (UK) Ltd is to recall all remaining unexpired stock of Nurofen Plus tablets of any pack size as a precautionary measure. The discovery of AstraZeneca's anti-psychotic drug Seroquel XL in Nurofen Plus packs on Aug. 25, 2011 was followed by a pack containing Pfizer's Neurontin 100mg, used to treat epilepsy, being found in Northern Ireland on Aug. 26, 2011.
The MHRA advised the public in a previous press release to return any packs of Nurofen Plus pain relief tablets to any pharmacy. The release also indicated the differences between the blister packs and medicines contained within. Seroquel tablets are large and capsule shaped in gold and black packaging, Nurofen Plus blister packs are silver and black, and Neurontin tablets are white capsules printed with name of the drug. Ian Holloway from the MHRA's Defective Medicines Report Centre (DMRC) said, "People should check to see if they have any packets of Nurofen Plus. If you do, return them to your nearest pharmacy."
Reckitt Benckiser issued the recall on the Nurofen website and stated, "Sabotage is suspected and we are working with the police on a formal investigation to find the person or persons responsible. Distribution of Nurofen Plus has been halted at this time."
NUROFEN PLUS NOW IN FULL RECALL 8-31-11
Drug alert: Nurofen Plus tablets update
Tue, 30/08/2011 - 11:32
By News team
All unexpired stock of Nurofen Plus tablets are now being recalled following further reports of Seroquel XL and Neurontin blister strips being found in cartons of Nurofen Plus.
The Medicines and Healthcare products Regulatory Agency issued a class 1 (patient level) drug alert on Friday evening (26 August 2011) following a recall by Reckitt Benckiser. The company has received further reports of two additional batches of Nurofen Plus tablets affected — one of the batches contained Seroquel XL tablets 50mg and one contained the Pfizer product Neurontin capsules 100mg.
Previous advice by the MHRA has been updated and Reckitt Benckiser is now recalling all remaining unexpired stock of Nurofen Plus tablets in any pack size as a precautionary measure. The MHRA is advising the public to return packs to pharmacies.
Enquiries related to Seroquel XL from AstraZeneca medical information on 0800 783 0033.
Enquiries related to Neurontin from Pfizer medical information on 01304 616161.
Enquiries related to Nurofen Plus from Reckitt Benckiser medical information department on 0500 455 456.
*This is only for the U.K.
United States Attorney General Eric Holder stated referring to AstraZeneca's Seroquel Settlement with DOJ: "These were not victimless crimes" April 27th, 2010
Wednesday, August 31, 2011
Monday, August 29, 2011
Weitz & Luxenberg P.C.'s Disgraceful AstraZeneca Seroquel Settlement Agreement Offer Commentary Part 4 - The Plaintiff Steam Roller
Weitz & Luxenberg P.C.'s Disgraceful AstraZeneca Seroquel Settlement Agreement Offer Commentary Part 4 - The Plaintiff Steam Roller
This is the final commentary installment related to the recent Seroquel settlement offer made by Weitz & Luxenberg that was sent to this blog by an anonymous party (which we can safely assume is a comparable reflection of for better or much worse; what other injured seroquel litigant victims will be receiving in short order from other various law firms across the country). It’s far past time that the actual victims of these kinds of corporate atrocities are heard in the court of public opinion for once (since "WE KNOW" it’s the actual victims of these corporate crimes who’s voices are seldom heard or reflected in mainstream media reports regarding huge tort litigation).
I have chosen to use a style of inserting opinion & commentary within the actual document using a different color *RED* & Italic font for this series of post.
The legal profession has (or least should have) an ethical, professional, legal, and moral responsibility to serve their clients “best interest” first and foremost when representing claims and/or actions taken against corporate malfeasance & crimes in these civil proceedings. If we didn’t know any better? One would get a idea that these offer documents read very much like they were written and pre-approved by AstraZeneca and their billion dollar legal dream team; instead of the plaintiff Attorney’s them selves?
Where we left off in Part 3 – a review
It is also important to note that even if your case is able to advance past this judicial scrutiny, a trial of this magnitude will cost over $750,000 per case and many of these costs are reimbursed off of the top of a favorable verdict reducing the amount received by the plaintiff. The ever popular legal version rendition of “Cry Me a River” Furthermore, any case that is won at trial will surely face a lengthy and hard-fought appeal. Now comes out the W&L patented & marveled crystal ball forecast that says even if you WIN, you will LOSE…Finally, nearly all of the Seroquel cases are being handled by three judges. Notice the fact that any mention of a jury is omitted… Even at an accelerated pace, the chances are slim that your case would be tried in the next 10 years if not longer. Once again the W&L legal crystal ball has predicted the worst possible scenario even decades out in one delectable morsel sleazy bite @ selling this bad deal to their clients….<Time for that puzzled befuddled look moment> who are W&L working for after all??? In taking all of these factors into consideration, and considering the issues of risk and reward, yours or the injured parties?…the deal they have hatched presents W&L no risk and rewards them quite handsomely…but what does it do for the injured parties they represent….the risk of horribly subverted justice, and the reward of a future with a poor medical prognosis mired in abject poverty…Sounds like a WINNER we believe that a settlement of this type offers you the most advantageous resolution possible. Insert: a loud verbose laugh track here….
Reading through this document I am actually astounded W&L would be even presenting such a huge pile of horse manure to those W&L are supposed to represent with professional and ethical standards of basic decency. This appears not to be the case with W&L; who no doubt have been joined by other corrupted and unethical ambulance chaser law firms from the across America in selling out their clients for their own corrupted personal gain…
I personally only ask that each of the injured parties take pause for one moment to remember the innocent fallen, the elderly, the children, our veterans, the disenfranchised and disadvantaged others that will never receive formal legal representation or their day in court as a direct result of AstraZeneca’s unconscionable acts and inhumane crimes related to the drug Seroquel..
Please take pause to do a serious heart and gut check; and come to the rational conclusion to do the right thing by REJECTING THIS HORRIBLE & UNFAIR SETTLEMENT OFFER
---------
---------
- Part 4
You will be notified once the Special Master Special Master? (Also known as a private hired gun)that will be used here so that W&L can bamboozle you into believing your getting a fair assessment and evaluation of your Case…no need for medical professionals, court determination of evidenced fact, an actual accounting of actual losses suffered, or a jury of your peers making such tedious determinations; that’s why W&L paid someone from the your settlement $$ pot to decide who gets what with a limited subjective criteria governed by an all mighty and infallible Injury Task Master…I’m sure W&L also were thinking about purchasing a batch of Lotto tickets for each of their clients, but then on second thought decided that would make this backroom rouse too obvious for all those sick and dying clients they were hanging out to dry for a nice tidy profit. issues your point award at which point you can appeal to the Special Master if you believe that your case involves extraordinary circumstances. Thereafter, the decision of the Special Master is final, binding and non-appealable. That right folks, once the special task master makes the unquestionable supreme ruling, you have no other options, say, or recourse…your arbitrary tablet point value commandment will be forever etched in stone or until Moses reappears to command that on a proclamation from GOD, W&L must set his people free.
We have retained the Garretson Law Firm to negotiate any and all Federal and State liens (Medicare/Medicaid/SSI) that may be applicable in your case. The Garretson Firm is a specialist in this field and they have obtained excellent results in their negotiation of similar liens in other litigations like Vioxx, Bextra, and Celebrex. Yes, W&L have taken the liberty to hire yet another outside resource that will be paid a nice chunk of change from the settlement pot to figure out how much of your already small TOKEN settlement will go directly to Uncle Sam & other greedy interested parties. I hope by now you realize as the injured easy mark; this is just the way Mass Tort works, everyone gets a slice of your pie, until finally at the end of some far off distant day in the future, you the injured or dying litigant receive a very small crumb leftover…WOW…where do I sign... Moreover, the Garretson Firm has already worked on thousands of other Seroquel cases and has been able to achieve excellent results in large part due to the volume of cases that they are able to include in a Global Resolution. At this time, the Garretson Firm has already negotiated with 44 individual states regarding Medicaid and they have reached an agreement to CAP Medicaid liens at a maximum of 20% of the settlement amount. Please see the enclosed blue booklet regarding governmental liens for more details. The details included any and all possible ways we can deduct more from your puny settlement offer…let’s briefly go through the list: deduct 33 1/3 % for W&L lawyers, Plus the W&L fees for all that time we sat around fancy resorts sipping martinis with the AZ legal dream team while coming up with this horrible offer for actual injured parties…. then another deduction for the Special Task Master, Yet another deduction for the Garretson firm, so they can determine how many other deductions they can find…Though we think that Uncle Sam will only want a mere 20% of your pittance Token….I think we are now getting the sharp painful thrust of this scam…that 12K track #1 in the end could look very much like a monthly utility bill rebate or an AZ RXSAVE discount coupon…Of course the other tracks are like the injured parties buying a blind passenger destination ticket on a train to nowhere. Additionally, please also be advised that if you or Weitz & Luxenberg are put on written notice of a private lien, for example from your medical provider, this lien must be resolved before any settlement proceeds can be distributed. If you have received any notice of a lien, please advise us immediately. Yes, the deduction gravy train doesn’t end there….unpaid parking ticket? Deduction…..medical bills made you declare bankruptcy? Deduction… ex-wife or husband wants their slice? Deduction…I think your beginning to get the point quite literally around that destination of where the sun don’t shine…
The terms of your signed contingency fee agreement will be in effect. We have taken great effort to keep the case specific expenses as low as possible. Insert: Laugh sound track Under the terms of your agreement, the contingency fee will be calculated after the expenses are deducted. Under the terms of our agreement with AstraZeneca, any interest earned on the settlement proceeds before allocation and payment to the individual clients will be used to offset the fixed costs of the Special Master and the Lien Administrator. Due to the low interest rates paid by all banks in this economy, we do not expect that there will be any leftover interest. However, in such a case, the leftover interest will be added to the settlement trust and will be distributed to all claimants as part of Judge Corodemus' allocation process.
After careful analysis of the likelihood of success in the court system, as well as the cost and time elements involved, we strongly recommend that you AGREE to participate in this settlement. We are confident that this settlement is the best possible resolution for W&L, trust us…this is a good pay day for W&L…we do this all the time…plus, if you don’t go along for the fleecing ride, we’ll crush you like a bug and send you packing anywise…that’s just what we do… of your claim especially in light of the history of this litigation. We expect that any case that does not agree to participate in this settlement will have a very difficult time navigating through the Court system and trial dates may not be set for many years. Moreover, as we have seen, these cases will be aggressively defended by AstraZeneca WHAT!!! did W&L actually believe in some seroquel induced delusional state that AstraZeneca was just going to walk up & hand over the keys to their massive treasure money vault, and say please treat those poor victims of our blatant criminal actions and poison pill to whatever you feel is an adequate relief for all the carnage, suffering, and damage we have inflicted? Is W&L actually trying to blow some patented “SMOKE&MIRRORS” con on their clients here, as they witnessed AstraZeneca gathering up a billion dollar legal dream team from just about every power house corporate law firm on the planet? Is W&L trying to scam their clients into believing that W&L didn’t know in advance that AstraZeneca was going to use every political favor card and legal trick available in this litigation to get cases tossed? What pathetic excuse is W&L going to throw at thier clients next? That this is so much different than all the other pharmaceutical tort cases you have profited from with excessive bounty to spare in the past….and we expect that we will receive motions to dismiss and unfavorable decisions from the Court on unsettled cases in the coming months. Moreover, if this settlement program is not successful (we fail to reach the 98% response rate) we highly doubt that we will ever obtain a settlement amount and terms as good as those contained in this program. That’s W&L way of saying…will dump you like a profitable bad habit suckers…take this TOKEN or fend for yourself…
While we hope that this letter and enclosures will help answer all of your questions, we understand that you may have additional questions for us. Please keep in mind that we are working very hard to evaluate each and every case individually and the many deadlines that are part of this settlement are fast approaching. Your case has been assigned to Judy Koenig. If you have an important question please call Judy Koenig at (800) 438-9786 and ask for extension 5860. Toll free number available for everyone….wonder if they have enjoyable background music playing while injured parties are sitting on hold waiting?….If you reach a voice-mail, please leave a message and you will be called back as quickly as possible. Please do not leave multiple voicemail messages on the same day as that will slow down our ability to return your call. Additionally, if you have email access, please try to email your detailed questions to us at sqinfo@weitzlux.com and we will get back to you quickly. If this letter has successfully answered your questions, and you have decided to participate, please carefully follow the directions on the front page of the enclosed release form and send your signed and witnessed release formes), your Track Selection AND your Brief Questionnaire to us as soon as possible. If you still have questions after reviewing this letter and after speaking to us over the telephone and you would like to meet us in person, we will be happy to schedule a meeting in our office at your convenience. Finally, please remember that the detailed information regarding this settlement program is COMPLETELY CONFIDENTIAL and should not be shared or discussed with anyone. It appears this rouse has somehow ended up not so COMPLETELY CONFIDENTIAL after all. Don’t you just hate that whole nasty concept of transparency, sunshine, and free speech…..how’s an ambulance chaser to run a sleazy profitable business model under those kinds of ridiculous & cumbersome impairments.
Jonathan Sedgh, Esq Glenn Zuckerman, Esq.
----------------------------------------------
If anyone has offer packets or information provided by their law firm or other related materials they would like to share here…please send them along in a publishable format through the email address provided on the side bar of this blog…***Please remember to remove all personal identifiable information from documents*** of course, unless you clearly wish or express that your correspondence include your identity….
Sunday, August 28, 2011
AstraZeneca's misleading commercial for Seroquel XR: this is an antipsychotic not antidepressant
The side effect list read includes having a doctor watch for cataracts (remember the beagle puppies study?they got cataracts)and marketing the antipsychotic for bipolar depression. The drug is an antipsychotic, not an antidepressant as the commercial misleads the public to believe with this DTC (Direct to Consumer) advertising.
AstraZeneca trialed Seroquel on beagle puppies: cataracts occurred at 4 times the human dose
AstraZeneca trialed Seroquel on beagle puppies:
"New generation atypical antipsychotic drugs may have an adverse effect on glucose metabolism, with hyperglycemia and a tendency toward development or exacerbation of diabetes.50–54 Clozapine and olanzapine may be diabetogenic, but for risperidone and quetiapine such associations are less clear. It remains controversial as to whether ziprasidone can influence glycemic control. There are findings that suggest the possibility of such alterations and reports that indicate no change in blood sugar assays.55 Alteration of glucose homeostasis from medicinal exposures may be due to pharmaceutical influences on various receptors, which change insulin and growth hormone physiology.50,51 If diabetic induction or diminished glucose tolerance were to occur in patients taking such medicines, it would be an additive risk factor for cataract development.
Quetiapine
This medicine was approved for marketing in the United States in 1997. There is concern that quetiapine might cause cataract formation.56 At four times the recommended human dose, studies with beagle puppies revealed cataract development. Even if this occurs in dogs, such effects in humans are not proven. Research on monkeys, at 5.5 times the recommended human dose, did not increase cataract development.56
Cataract occurrence has been observed in humans during long-term quetiapine treatment, but an etiological relationship between quetiapine and lens abnormalities has not been established. The presence of cataracts, even unrelated to any pharmaceutical use, increases with advancing patient age. Nevertheless, the possibility of lenticular pathology caused by quetiapine in humans cannot be excluded. Therefore, examination of the lens is formally recommended at initiation of treatment, or shortly thereafter, and at 6-month intervals during long-term therapy.56,57 This precaution is, however, not uniformly acknowledged.
A survey based on approximately 620,000 subjects was done to assess the risk of lens opacities in persons who had taken quetiapine in daily doses between 25 and 900 mg. Cataract formation occurred at a rate of about 0.005%, which is lower than the 0.2% incidence in the general population.58,59 A very low rate of lenticular opacities with quetiapine may be due to a limitation in the survey or insufficient data. This suggests that cataract occurrence associated with quetiapine treatment is probably not very common; however, the recommendation for regular eye examinations leaves a question about the risk involved. There is one case report of lenticular change developing while receiving quetiapine treatment; however, this individual was a smoker and received several medications that might have induced cataract occurrence.60 Coincidental occurrence could not be ruled out. A documented causal relationship is not evident in current literature. Ocular assessments may be based more on patient care and malpractice concerns than on objectified, proven pharmacological science. People with schizophrenia do have other risk factors, such as smoking, that contribute to cataract development. Any pharmaceutically induced tendency toward diabetes would be an additional concern.
Olanzapine
The manufacturer of this drug reports infrequent cataract formation observed in humans during treatment with olanzapine.61 The cause is unknown. Olanzapine is not implicated, but whether there could be any etiologic association between this medication and lens pathology has yet to be clarified. There are no formal recommendations to provide ophthalmologic examinations in patients prescribed olanzapine, but monitoring blood sugar levels has relevance as a diabetic precaution.61 There are concerns about glucose intolerance being associated with this medicine, but there is also evidence that documents no glycemic dysregulation with olanzapine.62
Clozapine and Risperidone
There is no evidence for clozapine being etiologically associated with cataract occurrence,63 nor is there such evidence for risperidone.64 Neither of these drugs mentions cataract occurrences in their prescribing information."
Also:
from Wikipedia:
The package insert fine print
13.2 Animal Toxicology and/or Pharmacology
Quetiapine caused a dose-related increase in pigment deposition in thyroid gland in rat
toxicity studies which were 4 weeks in duration or longer and in a mouse 2-year carcinogenicity study. Doses were 10-250 mg/kg in rats, 75-750 mg/kg in mice; these doses are 0.1-3.0, and 0.1-4.5 times the maximum recommended human dose (on a mg/m2 basis), respectively.
Pigment deposition was shown to be irreversible in rats. The identity of the pigment could not be determined, but was found to be co-localized with quetiapine in thyroid gland follicular epithelial cells. The functional effects and the relevance of this finding to human risk are unknown.
In dogs receiving quetiapine for 6 or 12 months, but not for 1 month, focal triangular cataracts occurred at the junction of posterior sutures in the outer cortex of the lens at a dose of 100 mg/kg, or 4 times the maximum recommended human dose on a mg/m2 basis. This
finding may be due to inhibition of cholesterol biosynthesis by quetiapine. Quetiapine caused a dose-related reduction in plasma cholesterol levels in repeat-dose dog and monkey studies;however, there was no correlation between plasma cholesterol and the presence of cataracts in individual dogs. The appearance of delta 8 cholestanol in plasma is consistent with inhibition of a late stage in cholesterol biosynthesis in these species. There also was a 25% reduction in cholesterol content of the outer cortex of the lens observed in a special study in quetiapine treated female dogs. Drug-related cataracts have not been seen in any other species; however,in a 1-year study in monkeys, a striated appearance of the anterior lens surface was detected in 2/7 females at a dose of 225 mg/kg or 5.5 times the maximum recommended human dose on a mg/m2 basis."
16 pages of AstraZeneca fineprint for Seroquel XR
Thursday, August 25, 2011
The Miller Firm LLC - AstraZeneca Seroquel Settlement - Legal Strong Armed Robbery? - Commentary
The Miller Firm LLC - AstraZeneca Seroquel Settlement - Legal Strong Armed Robbery? - Commentary
After mulling over this befuddling settlement offer paper work; I can only derive one simple conclusion. The Miller Firm LLC is virtually holding a gun to the heads of those permanently injured by the drug Seroquel. Of course as in line with the usual propaganda B.S., this document starts out saying "We are pleased to announce" that we have reached a settlement that "makes us" money and "makes you" go away.
What isn't stated in this offer document, is that it appears that THE MILLER FIRM LLC never intended to take a single case to trial, it also clear they never intend to now, or are they ever in the future when this settlement is REJECTED by plaintiffs.
In fact THE MILLER FIRM LLC appears to have never even investigated cases and collaborating /correlating evidence to determine which clients had a cases worthy of trial, or had they prepared even a single case...This is, and was always about gathering up as many cases as possible (even by ethically questionable means from other firms) and then going for a settlement pay day.
I personally have a very hard time seeing any place in this offer document where THE MILLER FIRM LLC seriously considered what was in the best interest of their clients. Again this law firm has crafted a document that appears was written by the opposition law firms representing AstraZeneca.
I would ask as you read this document; please pay close attention to how it tends to lean in one obvious prospective, while it hands over all the power and decision making to AstraZeneca (that same AstraZeneca that injured these thousands given all the power & control of injured parties settlement?). This document clearly reads by all intent & purpose as being a loaded gun held to the heads of injured parties; saying you must take this lousy offer or we'll send you packing.
So this is the kind of representation THE MILLER FIRM offers those that they are legally and ethically bound to serve in "THE CLIENTS BEST INTEREST".? ( not to mention pretty much every other firm that is playing tag along pay day in this litigation)
These horribly unfair settlement offers in a cross comparison quagmire spot light
92.5 million dollar distribution pot
2351 clients
(before deductions) average claim = 39 thousand & change
6.9 million dollar distribution pot
623 clients
(before deductions) average claim = 11 thousand & change
The same drug, same injuries, same tort, same defendant (AstraZeneca), and yet this huge difference in settlement offers to injured parties. None of the offers I have seen as of yet come anywhere close to being acceptable, fair or just; but something is horribly wrong & gone a rye with these extreme differentials between injury settlement offers in the very same litigation.
Honestly, I don't know how these ambulance chasers are able to sleep at night! I can only take a gander that lacking any moral conscience or sense of humanity has it's financial perks. I guess this document should really come as no surprise when you read correspondence between this law firm and their clients HERE .
Look at the numbers and ask yourself this question; if you had a life altering and life long medical condition that requires continuous treatment & monitoring, would five thousand dollars & change even come remotely close to covering those expenses? This is not to even mention quality of life issues, the pain, the suffering, loss of income, and a future that may hold out a fairly poor prognosis & early death.
Most people I know wouldn't call that a fair or a good deal....Now please take another look at the dollars figures in this document. Do you see that there are no deduction in this particular claim for medicare, medicaid, or other associated leans. When you begin to factor in those added deductions, you will find that many claimants will receive a mere small pittance from this offer. Maybe 3 thousands dollars if they get lucky in the calculations..less if they are not. Now please look up to the lawyers % and see how the lawyers will end up getting more than the actual injured parties.
So why should the average Joe American give a flying duck about the ramifications from this settlement? It doesn't effect you correct!
Unfortunately nothing could be further from the truth...It's you the American Tax Payer that will be footing the bill for all that medical care, & in some cases even food & housing from the actions AstraZeneca should be held responsible for. .
Now that is one slick deal that AstraZeneca and these law firms have worked out...AstraZeneca walks away unscathed; still making a tidy 5 & 1/2 billion dollars a year on Seroquel, while dumping all responsibility or accountability for their criminal actions and the path of carnage aftermath they have created on you Joe & Jane America. The Law firms pocket a nice tidy profit here too...what a deal...they win even if they lose ( a loss for their clients that is)...
Could it get any worse....you bet it can....I hope you realize Seroquel a powerful Antipsychotic drug is one of the most widely sold and prescribed drugs in America....How could that happen Just look here & find out....How about the sad fact that if we don't stand up and stop this run-away train robbery now....the health and financial consequences down the road will be unimaginably devastating...those hundreds of thousands of new damaged victims are unlikely to get legal representation....those are children, veterans, family members, your neighbor or loved one....
We have a huge problem & habit of waiting until a catastrophic crisis occurs in America before we take action...This problem can be faced and a resolution can be found now....or we as a society & nation will be forced to face it later..
----------------------------------------------------------------------------------------------------------------------
AstraZeneca Seroquel Litigation - Chicken Pluckers, Chicken Catchers, and the Plucked Seroquel Victims
Plaintiff Law Firms @ press conference announcing the great Seroquel Settlement Deal
AstraZeneca Seroquel Litigation - Chicken Pluckers, Chicken Catchers, and the Plucked Seroquel Victims
Though, I have to say that for myself and unquestionably for the vast majority of plaintiffs injured by this drug with irreversible conditions including diabetes and related ailments: an "envisioned big pots of gold" was never a thought that crossed my and others minds when we entered into this litigation process.
What we have asked for, and still do ask for; is an equatable & fair settlement or verdict, that would insure that victims of this drug received reasonable health care (now & in the future), recovery of actual losses, recognition of our pain and suffering, and the admission or a jury determination that indeed AstraZeneca & their Drug Seroquel with intended malice and forethought caused e-measurable suffering and injury to many tens of thousands of American citizens.
__________________
From Point Of Law .Com
Seroquel mass tort settlement?
August 25, 2011
Posted by Ted Frank
Alison Frankel is reporting claims that the last major chunk of the Seroquel litigation is settling confidentially at the nuisance rate of about $92 million for 2300 claims (after the first 25000 or so cases settled for an even smaller $647 million). These are figures that reflect avoiding the costs of defense rather than any risk of loss. Some plaintiffs who envisioned big pots of gold after being recruited by firms that advertised for Seroquel plaintiffs and then had their claims sold en masse to the chicken pluckers are disgruntled, but mass-tort litigation is designed to benefit the attorneys, rather than any plaintiffs, victims or otherwise. (Separately, I find it fascinating that attorneys are not allowed to hire runners to recruit clients—with the result that some attorneys effectively act as "chicken-catchers" indistinguishable from runners.)
Wednesday, August 24, 2011
AstraZeneca Seroquel - Marketing Death and Mayhem in pill form for profit - It's about you
AstraZeneca Seroquel - Marketing Death and Mayhem in Pill Form for Profit - It's about You
If you happened to read the post here on this blog prior to this, AstraZeneca's elite sales force: doctors, institutions, research organizations and politicians ;You, the reader should be able to develop a fairly good idea of how deep the influence peddling and corruption runs with corporations like AstraZeneca.
I can only surmise that most of the common folk in America may not fully conceptualize how this rampant out of control greed mongering effects them directly in their daily lives. The following article should hit home for many of those that serve this nation as active military, veterans, family, friends, and loved ones of those that serve or have served our nation.
They have offered America their sworn allegiance while preforming admirably the greatest patriotic sacrifice a citizen can give our nation . So what are they getting in return for this valiant service & sacrifice given freely with great honor to our country? They have been sold as a commodity "lab rats" to the forces of political wrangling, corporate greed, and institutionalized corruption.
VA Spent $717 Million on a Drug Deemed as Effective as a Placebo
By Bob Brewin, Nextgov.com
August 23, 2011
Over the past decade, the Veterans Affairs Department spent $717 million for an antipsychotic drug to treat post-traumatic stress disorder that a recent study shows is no more effective than a placebo.
Data provided by the department in response to a Nextgov query showed that VA doctors wrote more than 5 million prescriptions for risperidone from October 2000, the beginning of fiscal year 2001, through June 2010. Risperidone is the generic name for Risperdal, a second-generation antipsychotic drug originally developed by the Janssen Pharmaceuticals division of Johnson & Johnson to treat severe mental conditions such as schizophrenia and bipolar disorder.
But a paper by VA researchers published on August 2 in the Journal of the American Medical Association concluded, "Treatment with risperidone compared with placebo did not reduce PTSD symptoms."
That paper, whose lead author is Dr. John H. Krystal, director of the clinical neurosciences division of the VA's National Center for PTSD, concluded: "Overall, the data do not provide strong support for the current widespread prescription of risperidone to patients with ... military-related PTSD symptoms, and these findings should stimulate careful review of the benefits of these medications in patients with chronic PTSD." The study included 193 Vietnam War veterans and 63 veterans of the Iraq and Afghanistan wars.
Although the paper focused on risperidone, its authors also questioned the effectiveness of other second-generation antipsychotic drugs, including Seroquel, also known by its generic name, quetiapine. The VA told Nextgov that it spent $846 million on Seroquel over the past decade for more than 6.6 million prescriptions.
Besides prescribing Seroquel for use as an antipsychotic drug, both VA and Defense Department physicians prescribe it as a sleep aid, even though the drug has been implicated in the deaths of two Marines who took large doses.
The Food and Drug Administration has approved risperidone only for treatment of schizophrenia, bipolar disorder, and irritability associated with autistic disorder in children and adolescents, but clinicians are free to prescribe it as they see fit, a practice known as "off-label" use.
While the paper on risperidone published earlier this month reported the results of the first large trial measuring the effectiveness of second-generation antipsychotics in the treatment of PTSD, previous research has found little evidence that the drugs were effective; the VA's own clinical practice guidelines, first published in 2004, when the department spent $66 million on risperidone and $56 million on Seroquel, warned against using the medications to treat PTSD.
Those guidelines, still in force today for both the VA and Defense departments, cautioned, "There is insufficient literature to recommend the use of atypical antipsychotics (olanzapine, quetiapine, risperidone, ziprasidone, aripiprazole) to recommend their routine use in PTSD."
Douglas Leslie, a professor in the Penn State College of Medicine's public health sciences department, conducted a study of 279,778 veterans who had received off-label prescriptions for antipsychotic drugs for PTSD treatment in 2007. The veterans were most often prescribed Seroquel, followed by risperidone.
In a 2009 paper, he concluded, "The scientific evidence supporting the effectiveness of off-label antipsychotic medication use is weak for most conditions, including depression and PTSD -- conditions with relatively high rates of off-label use in our study."
In 2007, the year that Leslie was conducting his research, the VA spent $89 million for 467,217 risperidone prescriptions and $92 million for 740,317 Seroquel prescriptions.
Teresa Mueller, a Janssen spokeswoman, said, "The treatment of post-traumatic stress disorder with Risperdal [risperidone] is not approved by the FDA, and as such, we do not promote its use for this medical condition."
But the company is under investigation by the Justice Department and several state attorneys general for its sales and marketing practices regarding Risperdal, according to Johnson & Johnson's quarterly report filed on July 3 with the Securities and Exchange Commission.
According to the report, the company in 2004 "received a subpoena from the Office of the Inspector General of the United States Office of Personnel Management seeking documents concerning sales and marketing of, any and all payments to physicians in connection with sales and marketing of, and clinical trials for, Risperdal from 1997 to 2002. Documents subsequent to 2002 also have been requested by the Department of Justice."
The Johnson & Johnson report also identifies lawsuits filed by eleven states related to sales and marketing of Risperdal for off-label use.
Subscribe to:
Posts (Atom)